-
+ Oxygen Concentrator
Portable Oxygen Concentrator Medical Oxygen Concentrator+ PAP Ventilator
Sleep Treatment COPD Treatment+ Nebulizer
Compressor Nebulizer Pulsating Nebulzier Mesh Nebulizer
About Resplev
Resplev focuses on the research and development of medical devices in the areas of respiratory and sleep health, and provides high-performance and cost-effective devices for the lung and nasal sinuses inhalation treatment, domestic ventilation, and oxygen generation.
As a professional medical manufacturer ,Resplev is headquartered in Suzhou National New&Hi-Tech Industrial development Zone. We have an independent R&D center, two manufacturing plants and quality inspection department. Products have obtained China NMPA, CE certification. Its integrated medical solutions and customized service have won customers in many countries and regions.



Portable Oxygen Concentrator
The battery lasts for 5 hours;
Medical oxygen flow Max 1200mL;
Compact design, weighing just 2.3 kg;
Detachable design of molecular sieve.

Sleep Treatment
Accurately identify multiple respiratory events
Steady adjustment of ventilation strategy for higher compliance
Self adjusting pressure regression under different masks in the market
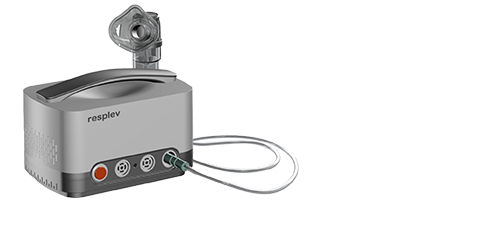
Pulsating Nebulzier
Differentiable pulse frequencies for children and adults
Nebulized particles mainly deposit in the maxillary/ethmoidal/frontal sinuses
Ultra low power consumption/oil-free motor, 5-year warranty for household use
Support and Service
-


Qualification Advantages
Resplev has independent research and development, manufacturing, and quality inspection departments, and its products have successively obtained registration and certification from China's CFDA, EU CE, and US FDA.
-


Team Advantages
The core team of R&D, clinical and after-sales has over 10 years of experience in the field of respiratory therapy, providing one-on-one services to more than 10000 users.
-


Technical Advantages
We have over 30 independent intellectual property rights covering various dimensions such as appearance, structure, algorithm, and cloud interaction.
Events
-

2024-10-30
Guidelines for home oxygen therapy for adult respiratory diseases in China(2024)
View More
-
2024-09-01
Expert consensus on rational use of nebulization inhalation therapy(2024)
View More
-

2019-06-16
Nebulizer and atomization parameters(2019)
View More
-

2018-09-20
Expert consensus on emergency oxygen therapy(2018)
View More








